

Mitotic catastrophe has well been described as a cause of cell death after premature onset of mitosis with unrepaired DNA damage, which is associated with a defective G2/M checkpoint ( 11). In the latter cases, cells exit mitosis before execution of the cell death pathway by eventual satisfaction of the SAC, or by gradual degradation of cyclin B in the presence of an active SAC, which allows for anaphase, telophase, and cytokinesis to be omitted ( 10). One of the mechanisms that is associated with some degree of mitotic arrest owing to a failure to proceed with prometaphase or metaphase, is known as mitotic catastrophe, which has at least three different consequences: cell death during mitosis, cell death after mitotic exit, and senescence after mitotic exit ( 9). Indeed, the outgrowth of tetraploid cells is inhibited by tumor suppressors, p53 and Rb, which are frequently inactivated in tumors carrying abnormal hyperploid karyotypes ( 8). These mechanisms are considered to be tumor suppressive because tetraploid and aneuploid cells are potentially tumorigenic ( 5– 7). In a wild type genetic background, cells with abnormal ploidy rarely outgrow owing to mechanisms, by which cells that spend an abnormal time in mitosis are removed from the population. Tetraploid cells generated through cytokinesis failure are relatively unstable compared to their diploid counterparts and frequently become aneuploid upon continued cell division ( 4).
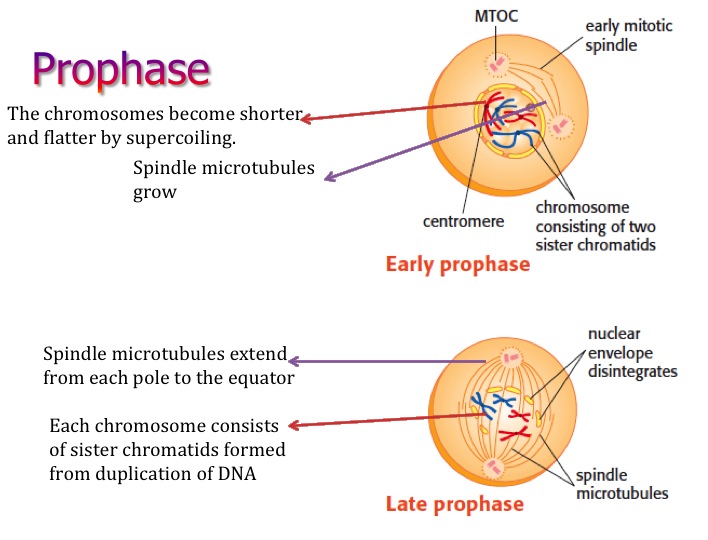
Then the nuclear envelope is reformed around the sets of daughter chromosomes during telophase, which is followed by cytokinesis, a distinct process that pinches off the separated daughter chromosomes via the cleavage furrow.įailures during the mitotic process or cytokinesis potentially cause the formation of cells with abnormal ploidy, such as aneuploidy or tetraploidy. During anaphase sister chromatids split away from each other by the force of microtubules and start decondensation when telophase begins. Released and activated separase cleaves cohesin that holds sister chromatids together, which leads to the onset of anaphase. Destruction of cyclin B inactivates CDK1, the activity, which inhibits reactions required for mitotic exit, while securin destruction activates its target protease, separase. Consequently, the anaphase promoting complex (APC), a ubiquitin ligase that induces destruction of its critical substrates cyclin B and securin ( 3), is activated. When all sister chromatid pairs are aligned on the spindle equator, with all sister kinetochores properly attached to the spindle, the SAC is satisfied. To avoid premature separation of sister chromatids, the spindle assembly checkpoint (SAC), which monitors mitotic progression during this time, is active until metaphase. Nuclear envelope breakdown (NEBD) terminates prophase and initiates prometaphase, during which microtubules emanating from separated sister centrosomes find and attach to kinetochores, a huge protein-DNA structure formed at the centromere ( 2). Mitosis starts when condensed chromosomes become visible in the prophase nucleus. Although an alternative definition of mitotic progression based on the changes in cell-cycle regulators has been proposed ( 1), we here apply the traditional definition based on the morphological change of typical vertebrate cells, since it is more relevant to the focus of this review. Mitotic progression is associated with a highly dynamic change of chromosome morphology and movement, which is traditionally classified into 5 stages: prophase, prometaphase, metaphase, anaphase, and telophase.


 0 kommentar(er)
0 kommentar(er)
