
Add urea until the solution is saturated. Stir the solution using a magnetic stirrer until the solid is completely dissolved.
PRECIPITATE COLOR SOFTWARE
Visolve is a software application (free for personal use) that transforms colors of the computer. Filter the solution through a Buchner flask and scrape the solid into a beaker containing 200 cm3ammonia solution (2 mol dm-3). This can be a problem for people with color vision deficiency.

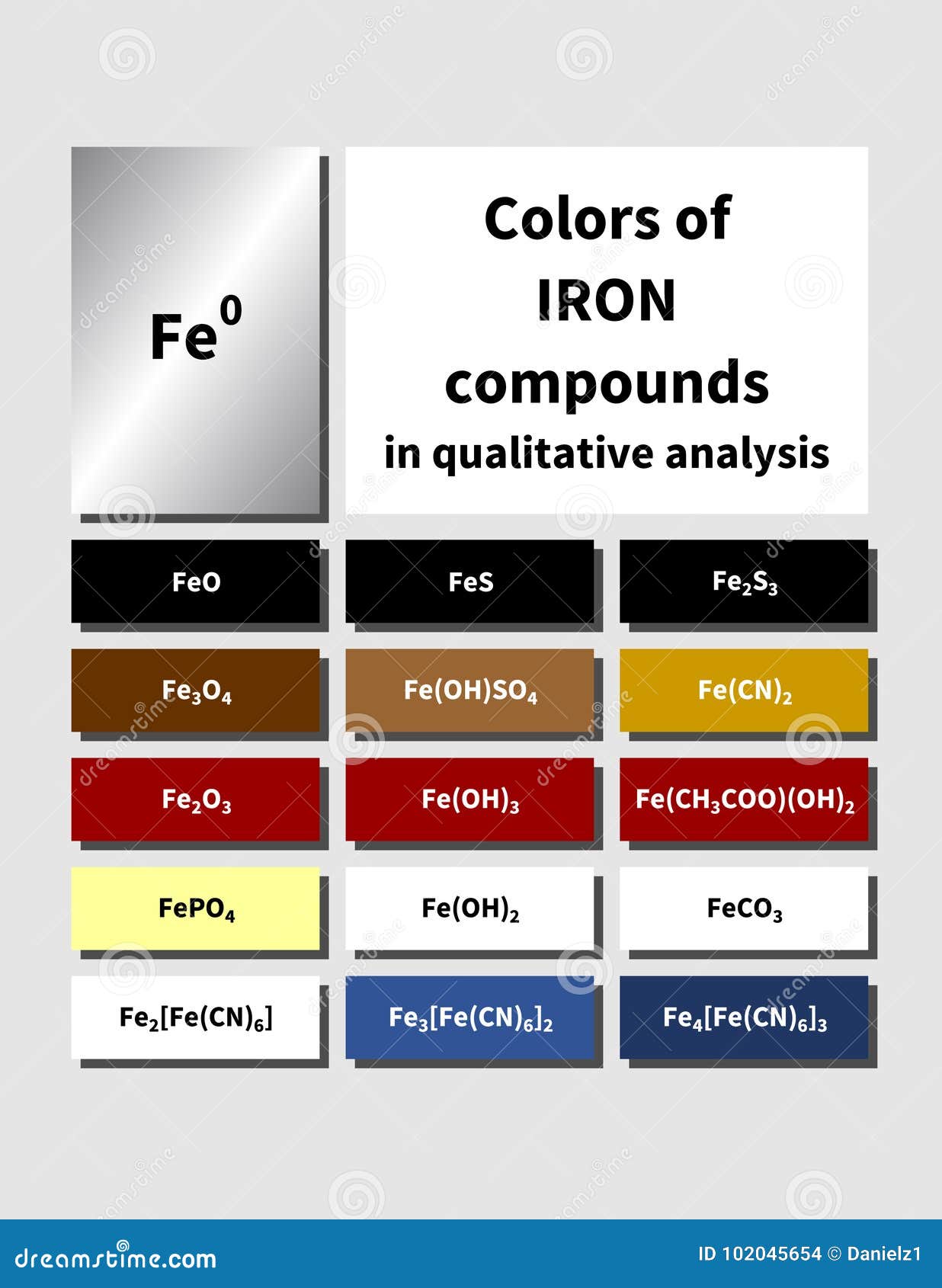
Since the compound forms an insoluble precipitate with hydroxide, it eliminates the alkali metals. This is to avoid black stains on the tooth. An orange-brown precipitate will form by double decomposition. The color of the solid is characteristic of the Fe3+ ion. The addition of solution (2) is to remove the excess silver ions that may form the dark silver phosphates. So, the possible by-products could be Calcium ions, fluoride ions, ammonium ions, silver ions, silver phosphate. The silver ions also react with the tooth structure to form the dark black silver phosphates. It has been postulated that the fluoride usually reacts with hydroxyapatite, by replacing the calcium to form fluoroapatite, while the silver diamine exerts an antibacterial effect. When solution (1) is first applied to the tooth, the high pH has been shown to aid in the formation of covalent bonds of phosphate groups onto proteins and crystallites to grow. Tooth: Consists of collagen, proteins, hydroxyapatite, bacteria. Addition of OH- ions to metal-aqua ions, by dilute sodium hydroxide (NaOH) or dilute ammonia (NH4), results in the formation of insoluble precipitates. Solution (2): Potassium iodide has a pH of 7. Solution (1): Silver diamine fluoride has a pH of 10. No new substance gets formed in these reactions. Riva Star comes in 2 solutions that are mixed together in the tooth cavity. Explanation:- A physical reaction is defined as the reaction in which a change in shape, size takes place. There is some cool chemistry going on here but I am unsure what it is. The excess solution in the tooth cavity is then rinsed off with water. AgCl is a white precipitate and AgBr is a light yellow precipitate. It appears good before autoclaving but often gets precipitated (white in color) after autoclaving. To add further to your question, for a silver diamine dental product (Riva Star by SDI), the white precipitate disappears after applying more potassium iodide to the mixture. I am regularly preparing 1/2 MS liquid media for transformation protocol. I assume that the original silver solution was the diammine complex to prevent silver chloride from precipitating as soon as chloride ions are added (due to not perfectly deionised water being used). The yellow precipitate that forms is silver(I) iodide, $\ce$$


 0 kommentar(er)
0 kommentar(er)
